Abstract
To date, there are few, if any, behavioral or exercise interventions that have been shown effective in improving cognitive functioning in adolescents with Down syndrome (DS). Exercise is a logical answer because it has been repeatedly shown to improve cognitive and physical and mental health in typical populations. However, current exercise recommendations for persons with DS vary greatly. Recommendations are often nonspecific in terms of the type or intensity of exercise, and results on improvement of cognitive functioning are equivocal. This chapter will report on preliminary data of an 8 week intervention of assisted versus voluntary cycling exercise on cognitive and health functions in adolescents with DS. Assisted Cycling Therapy (ACT) is innovative and important because it is predicted to enhance neurogenesis, which in turn may improve multiple central nervous system functions related comorbid conditions in adolescents with DS.
1. Introduction
Down syndrome (DS) is one of the most prevalent chromosomal conditions, affecting 1 in every 691 live births in the U.S.A. [1]. One of the main features of DS includes cognitive impairments. Specifically, adolescents with DS have been shown to have lower levels on executive functions including working memory, inhibition, planning and set switching than typically developing children matched for mental age [2]. These cognitive deficits can limit their abilities to perform activities of daily living. Thus, interventions to improve their capacity to perform activities independently would help to improve quality of life and reduce the costs associated with providing care for them. We are investigating the effects of physical activity interventions on the cognitive skills in persons with DS.
1.1. Executive function
Executive functions are a set of higher-order control processes that take place primarily in the frontal lobe of the brain [2], which deal with the decisions to make actions, and planning how to accomplish tasks [3]. Executive function includes concept formation, task switching, inhibition, volition, planning, purposeful action, and effective performance [3]. These are necessary in order for a person to engage in tasks independently. People with deficits in executive function are often called lazy due to this lack of initiative, but executive function is necessary for a person to initiate self-care routines or work independently. Many researches have documented that people with DS have shown deficits in executive functioning (e.g., [4, 5]). Improving executive functions could in turn improve many other independent living skills. Below we have highlighted a few executive functions that we measured in response to an exercise intervention in persons with DS.
1.2. Working memory
Working memory is information that people actively keep in their mind and manipulate [3, 6]. If human memory were a computer, the working memory would have been an active window where a person would have manipulated the informational contents. Working memory is limited in size, yet it is important for many other tasks from remembering words to learning new motor skills [6, 7]. Several studies have found that people with DS have significant deficits in working memory [8, 9].
1.3. Set switching
Set switching is the ability to change a course of thought or action based on changing requirements [3]. In clinical settings, this is typically done with a card sorting test where children are first asked to sort the cards by shape and then by color. Children with typical development are unable to switch to the second sorting rule at three years old. By four years of age, a child can change rules with some struggle, and by five years old, a child can shift to the new rule with ease [10]. On a practical level, set switching is demonstrated while children are working on something when a parent tells them that they need to get ready to leave the house. The ease at which the children are able to transit between the two tasks reflects their capacity for set switching. Set switching also requires working memory to process the change in tasks and the ability to inhibit the first behavior pattern [11]. Set switching activates a network of cells in the frontoparietal region of the brain, including the inferior frontal gyrus, anterior cingulate cortex, and supramarginal gyrus [12]. When someone has difficulty with set switching, it can result in inflexibility and perseverative behaviors. People with DS have significant deficits in set switching in comparison to people with typical development [2, 8].
1.4. Verbal fluency
People with DS in general show deficits with language, especially with expressive vocabulary [13–15]. Typically this is tested by asking people to recall words related to a particular category or words that start with a certain letter. Neuroimaging studies have shown that letter-based verbal fluency is mediated by the frontal cortex and category-based verbal fluency by the temporal cortex; parietal lobe mediates both tasks [16]. Nash and Snowling [17] found that people with DS showed deficits in verbal fluency in comparison with peers of typical development.
As previously described, there is a vast amount of research that documents cognitive deficits in persons with DS. We believe that it is time to focus on interventions aimed at improving cognitive functions in persons with DS. Our innovative exercise intervention and results will be explained next.
2. Intervention: Move fast, think fast
Exercise is a logical intervention for effective treatment of cognitive impairments in persons with DS because the positive influence of voluntary exercise on cognition has been demonstrated in other typical populations [18, 19], including children [20, 21] and older adults [22, 23]. Furthermore, voluntary exercise has been shown to improve memory in mice models (Ts65Dn) of DS [24]. However, a recent review of the therapeutic benefits of exercise in persons with DS found that exercise was nonsignificant in improving physical and mental health outcomes in persons with DS [25]. Because persons with DS move slowly [26] due to slower reaction times [27], deficits in muscular strength [28], and reduced cardiorespiratory capacity [29], adolescents with DS typically ‘do’ not exercise at a relatively high rate, ‘thus, they miss out on the opportunity to gain’ cognitive improvements through neuroplasticity in the brain. Furthermore, approximately 61% of persons with DS have been shown to have low exercise tolerance [30] which reduces their exercise time and intensity and which seems to limit the cognitive benefits of exercise for persons with DS [25]. The
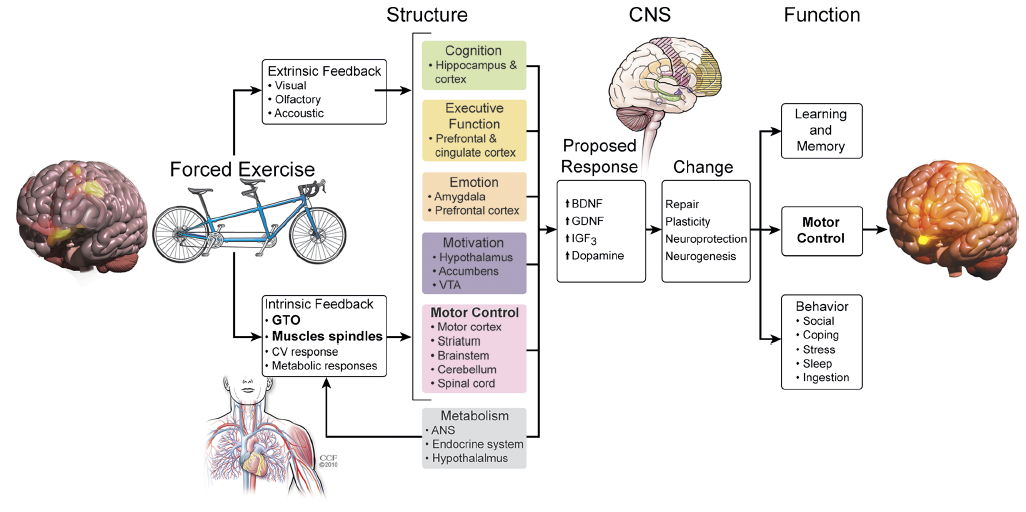
There is an emerging body of literature in healthy older adults and individuals with Alzheimer’s disease indicating that exercise results in structural and functional changes in the brain [31]. These alterations in brain structure and function suggest that CNS function can be altered via voluntary exercise in individuals with relatively normal and abnormal patterns of activation within the motor cortex. However, because persons with DS have limited motor output due to physiological and psychosocial factors, their ability to induce changes in CNS function may be compromised when engaging in voluntary exercise performed at their preferred (i.e., low) rates. They may need to have exercise augmented through mechanical assistance as proposed in our assisted exercise paradigm, coined Assisted Cycling Therapy (ACT) in 2013 [32]. Assisted exercise is an approach initially used with animals which were exercised on a motorized treadmill at a rate greater than their voluntary exercise rate. Assisted exercise has demonstrated improvements in cognitive functioning in animals [33] and most recently in patients with Parkinson’s disease [34, 35]. ACT has been suggested to improve motor and cognitive function through its neuroprotective properties as demonstrated in Figure 1, a model proposed by Alberts and colleagues [34].
2.1. Procedures for assisted cycling therapy
ACT is an emerging exercise paradigm especially suited for clinical populations who have limited voluntary movement output, exercise capacity, or exercise motivation. During ACT the electric motor of the bicycle is engaged which helps to increase pedaling cadence to a predetermined rate. We have used absolute cadences of approximately 80 rpm or relative cadences of 35% greater than the initial voluntary pedaling rate. The initial pedaling rates, however, may need to be increased gradually for comfort and familiarization. The ACT condition often leads to reduced power compared to voluntary pedaling as indicated in Table 1 by the lower average power contribution of our participants in the ACT condition than the voluntary cycling condition. As can be seen in Figure 2, special procedures were utilized to ensure that the feet were not positioned too far forward and that they did not slip forward, side-to-side, or backward to ensure a high degree of safety at the high pedaling rates.

The length of our intervention period was eight weeks with three cycling sessions per week. Before each cycling session, the resting heart rate (HR) was obtained while the participant was sitting on the bike. A five-minute warm up at a voluntary rate was completed before the 30-minute cycling session regardless of the condition (ACT or voluntary cycling (VC)). On the first day, the average cadence from the warm-up period was multiplied by 1.35 to determine the initial ACT cadence. This step was omitted in the voluntary cycling condition. Thus, the cadence on the first day of the ACT intervention was set at a rate which was 35% faster than the voluntary cadence. A three- to five-minute cool-down at the end of the ACT or voluntary cycling session was optional. During the cool-down, the motor was not engaged. The average HR (bpm), cadence (rpm), and power (Watts) was recorded every five minutes during the cycling session (refer to Table 1 for mean values). These averages did not include the warm-up period.
To monitor Rate of Perceived Exertion (RPE), we used a modified -point RPE scale. A rating of 2 or 3 on the -point RPE scale (1, easy/not-tired; 2, a little hard/a little tired; 3, hard/tired; 4, very hard/very tired) was desired to keep the exercise intensity at a moderate level. The goal was for participants to cycle between 64 and 76% of their age-predicted maximal HR (HRmax = 210–0.56×age–31, [36]) which corresponds with a moderate exercise intensity as dictated by the American College of Sports Medicine [37]. Thus, for most participants in the ACT condition, we increased cadence from session to session by 3–5 rpm, based on tolerance, up to the maximum cadence of the motor (e.g., 95 rpm) or until 64% of age-predicted HRmax or a personal tolerance limit was reached. Participants in the ACT group took on average 13.2 cycling sessions to reach this point. Participants in the voluntary cycling group were not encouraged to pedal faster as the goal was to have them exercise at their preferred voluntary rate (refer to Table 1 for cadence values).
For this randomized control trial, participants were randomly allocated to eight weeks of ACT, eight weeks of VC, or eight weeks of no cycling (NC). The ACT and VC conditions were described in the previous section. Participants in the NC group completed only the pre- and post-testing sessions separated by eight weeks and they were instructed not to change their usual physical activity habits and therapy regiments for the eight weeks. Inclusion criteria consisted of trisomy-21 and a chronological age of 9–26 years. Exclusion criteria consisted of other genetic conditions and neurological disorders (e.g., ADHD and autism), medical contraindications to exercise, and sensory or physical impairments which preclude completion of the cycling intervention. During the pretesting sessions (first visit to the laboratory), the participants’ height, weight, vision, hearing, and mental age were recorded or assessed. Mental age was determined with the Peabody Picture Vocabulary Test (4th ed.; [38]) (refer to Table 1 for chronological and mental age values). In addition, all participants had functional hearing and vision for the purpose of the testing procedures. Then, three executive function tests were administered in random order.
3. Measures
The verbal fluency test consisted of four categories: animals, food and drinks, words that start with an S, and words that start with an F. The participants were given one minute per category and had to name as many words in the category as possible. The verbal fluency test was a test of verbal long-term and working memory, attention, and inhibition [39, 40]. As mentioned, verbal fluency and other speech and language deficits are well documented in persons with DS [41–43]. Verbal fluency tests have been used as behavioral measures of hippocampal and prefrontal cortex function [40, 43].
A backward digit span test was administered as a behavioral measure of working memory, which requires the simultaneous storage and processing of information [6, 44]. It is considered a prefrontal function [6]. During the backward digit span test, participants had to reverse a sequence of numbers given by the investigator. The investigator was providing progressively longer sequences of numbers until the participant could no longer accurately articulate the given sequence in reverse order.
The Wisconsin Card Sorting test (modified for DS) measures set-switching ability and working memory which are functions of the frontal cortex and parts of the parietal lobe [45, 46]. In this task, the participants are asked to match either shapes or colors with rule changes taking place during testing. Adolescents with DS have been found to have reduced capacity for set switching compared to typically developing adolescents [2]. These three executive function tests were repeated during post-testing.
4. Results
Cohen’s
5. Interpretation of results
It is clear that cycling exercise, whether it is assisted or voluntary, is more beneficial to executive function than no exercise. However, ACT seems to be more effective in improving working memory, whereas VC seems to be more effective in improving verbal long-term memory, and set switching than ACT.
Based on our results, a moderate exercise intensity, of between 64 and 76% HRmax, may not be necessary for benefits as the average HR during ACT or VC cycling sessions was just below 64% of the average age-predicted HRmax. The average chronological age of our participants in the ACT and VC group was 19.4 years and 18.4 years, respectively. This translates to minimum average target HRs (64%) of 107.6 bpm and 108.0 bpm in the ACT and VC group, respectively. In addition, their average exercising target HR of 98.7 bpm and 100.7 bpm were below the target HR range. In fact, on the first day of cycling, only 11% of ACT and 31% VC participants reached 64% of their age-predicted maximal HR. The only difference between the ACT and VC groups was the cadence at which they were cycling. We can thus conclude that the specific adaptations in terms of executive function are due to the different rates of movement.
The greater movement frequency during ACT would presumably lead to more frequent stimulation of the Golgi tendon organs and muscle spindle fibers in the lower extremity musculature and associated tendons, which in turn translates to greater afferent input to the frontal motor cortex [34]. This greater stimulus frequency in turn seems to be necessary to maximize benefits to working memory but does not seem necessary to improve long-term memory recall, attention, or set-switching ability. As can be seen in Table 1, heart rates, and therefore cardiovascular workloads, were similar between ACT and VC, the only plausible explanation that remains for these group differences is the voluntary movement output during VC. The voluntary activation of certain areas of the motor cortex may thus be unique to voluntary exercise or greater in magnitude than the afferent stimulation resulting from ACT and thus benefit the frontal cortex in specific ways.
Differences in performance among executive function tasks, as observed in this study, have been documented [48]. Our results also suggest that different executive functions (e.g., working memory, attention, inhibition, and set-switching), though all mediated by the frontal cortex, may differentially benefit from different modes of exercise.
WRITTEN BY
Submitted: October 2nd, 2014 Reviewed: April 15th, 2015 Published: September 2nd, 2015
DOI: 10.5772/60636
"CITE"
Ringenbach, S. D. R. , Holzapfel, S., Pandya, G. M. M. a. , 2015, 'Assisted Cycling Therapy for Persons with Down Syndrome — Implications for Improvements in Cognitive Functioning', in S. Dey (ed.), Health Problems in Down Syndrome, IntechOpen, London. 10.5772/60636.
https://www.intechopen.com/chapters/48560

